Lead Compound TTI-0102
TTI-0102 is a novel prodrug of cysteamine classified as a New Chemical Entity (NCE). It possesses an asymmetric disulfide configuration, composed of cysteamine and an additional thiol component. TTI-0102 treats diseases linked to mitochondrial oxidative stress by gradually releasing cysteamine in two metabolic steps. This reduces side effects, allows higher doses, and may enable once-daily use compared to earlier therapies.

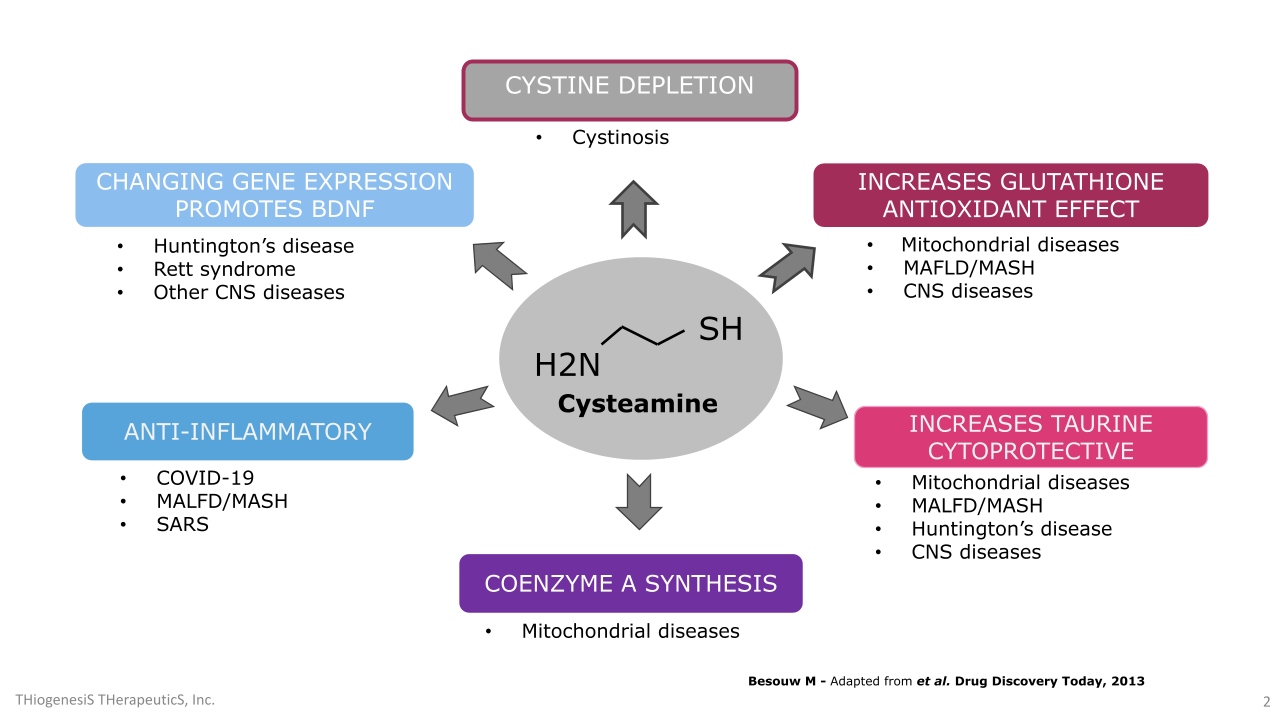
TTI-0102 and cysteamine uniquely boosts intracellular cysteine, which drives the production of glutathione, taurine, and coenzyme A: three molecules essential for mitochondrial redox balance, energy metabolism, and cellular protection.
TTI-0102 is currently being evaluated in multiple Phase 2 programs, including an active trial in MELAS (EU) and a cleared IND for Leigh syndrome spectrum (U.S.), with additional trials in pediatric MASH and Rett syndrome in preparation.
TTI-0102 - Phase 1 Study
TTI-0102 was evaluated in a Phase 1 dose-escalation trial with healthy volunteers, assessing its pharmacokinetics and tolerability against generic cysteamine. Doses reached up to 2400 mg cysteamine equivalent (four times the standard 600 mg), with results showing:
- TTI‑0102’s cysteamine Cmax did not surpass that of 600 mg generic cysteamine,
- Therapeutic levels lasted over 24 hours, potential for once-daily administration.
- Only mild body odor occurred at the highest dose; no GI side effects were reported.
These findings suggest that TTI‑0102 reduced the spike in cysteamine associated with its side effects; offering improved tolerability, a significantly increased dosing range, and the potential for once-daily administration compared to generic cysteamine.

Cysteamine

Cysteamine is a thiol compound that has been studied for decades for its antioxidant, anti-inflammatory, and neuroprotective properties. It works in part by boosting intracellular cysteine level, the rate-limiting precursor to glutathione, and is also metabolized into taurine and Coenzyme A (“CoA”). These compounds are central to maintaining redox balance and defending cells from oxidative damage, particularly in mitochondria.
Glutathione is the body’s most abundant intracellular antioxidant. It protects against oxidative stress by neutralizing reactive oxygen species (“ROS”), supporting enzymatic activity, and recycling other antioxidants. Taurine modulates antioxidant enzymes, stabilizes mitochondria, and supports calcium signaling. CoA is a vital cofactor in cellular energy metabolism and has been shown to protect cells from oxidative or metabolic stress.
Cysteamine was first approved in the 1990s to treat nephropathic cystinosis, a rare lysosomal storage disease, where it helped reduce cystine accumulation in tissues. Despite its proven efficacy, cysteamine’s broader therapeutic potential has been limited by tolerability issues, namely its short half-life, dose-limiting gastrointestinal side effects, and strong body odor associated with sulfur metabolism.
Thiogenesis’ lead candidate, TTI-0102, is a next-generation cysteamine prodrug designed to overcome these limitations. By improving cysteamine’s delivery and pharmacokinetics, TTI-0102 aims to unlock the full therapeutic potential of this well-characterized molecule across a broader range of mitochondrial and oxidative stress-related diseases.

Cysteamine - Mechanisms of Action
Relevant Publications - Available Online
- Akerfeldt, S. (1963). Radioprotective Effects of S-Phosphorylated Thiols. Acta Radiologica: Therapy, Physics, Biology, 1(6), 465–470. https://doi: 10.3109/02841866309134122
- Besouw, M., Blom, H., Tangerman, A., Graaf-Hess, A. de, & Levtchenko, E. (2007). The origin of halitosis in cystinotic patients due to cysteamine treatment. Molecular Genetics and Metabolism, 91(3), 228–233. doi: 10.1016/j.ymgme.2007.04.002
- Guha, S., Konkwo, C., Lavorato, M., Mathew, N. D., Peng, M., Ostrovsky, J., … Falk, M. J. (2019). Pre-clinical evaluation of cysteamine bitartrate as a therapeutic agent for mitochondrial respiratory chain disease. Human Molecular Genetics. doi: 10.1093/hmg/ddz023
- Paul, B. D., & Snyder, S. H. (2019). Therapeutic Applications of Cysteamine and Cystamine in Neurodegenerative and Neuropsychiatric Diseases. Frontiers in Neurology, 10, 1315. doi: 10.3389/fneur.2019.01315
- Pozzo-Miller, L., Pati, S., & Percy, A. K. (2015). Rett Syndrome: Reaching for Clinical Trials. Neurotherapeutics, 12(3), 631–640. doi: 10.1007/s13311-015-0353-y
- Schwimmer, J. B., Lavine, J. E., Wilson, L. A., Neuschwander-Tetri, B. A., Xanthakos, S. A., Kohli, R., … Yates, K. (2016). In Children With Nonalcoholic Fatty Liver Disease, Cysteamine Bitartrate Delayed Release Improves Liver Enzymes but Does Not Reduce Disease Activity Scores. Gastroenterology, 151(6), 1141. doi: 10.1053/j.gastro.2016.08.027
- Skrede, S., & Christophersen, B. (1966). Effects of cystamine and cysteamine on the peroxidation of lipids and the release of proteins from mitochondria. Biochemical Journal, 101(1), 37–41. doi: 10.1042/bj1010037
- Thoene, J. G., Oshima, R. G., Crawhall, J. C., Olson, D. L., & Schneider, J. A. (1976). Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo. J Clin Invest, 58(1), 180–189. doi: 10.1172/jci108448
- Wang, Y., Davis, I., Chen, Y., Naik, S. G., Griffith, W. P., & Liu, A. (2020). Characterization of the non-heme iron center of cysteamine dioxygenase and its interaction with substrates. Journal of Biological Chemistry, jbc.RA120.013915. doi: 10.1074/jbc.RA120.013915
