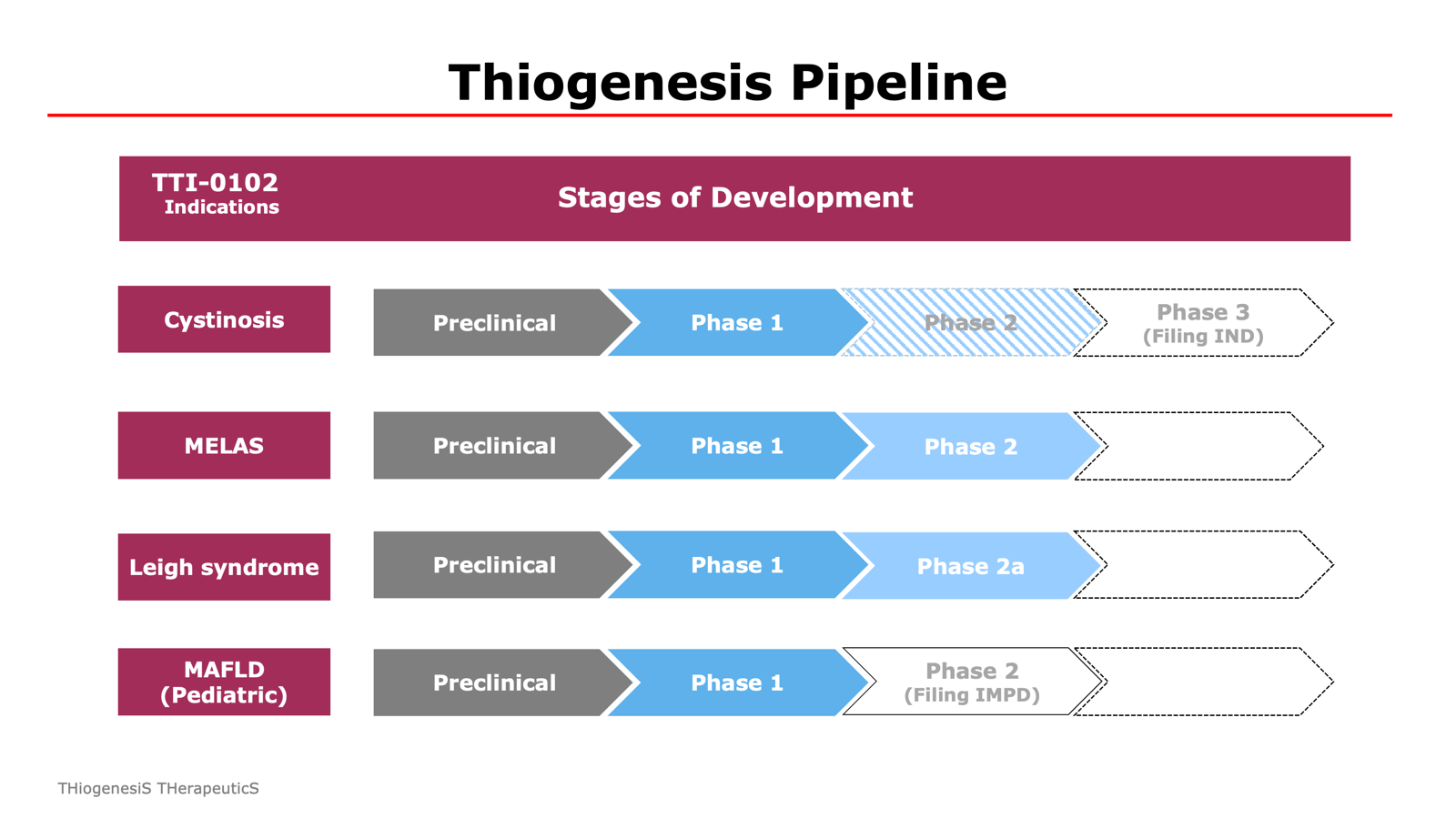
TTI–0102 Pipeline
(Pediatric)
Regulatory
The Food and Drug Administration (FDA) and the European Medicines Agency (EMA) will govern Thiogenesis’ clinical trials in their respective jurisdictions. As a prodrug, TTI-0102 is eligible to use the expedited 505(b)(2) regulatory pathway in the US and its equivalent hybrid pathway in Europe. The 505(b)(2) pathway provides certain regulatory advantages, as some of the clinical safety data from the active compound may be provided by referencing previous studies not affiliated with Thiogenesis (in this case, safety data from Cystagon®), which can save substantial time and money in progressing to human efficacy trials.
About Nephropathic Cystinosis
Nephropathic cystinosis is a rare, inherited lysosomal storage disorder caused by mutations in the CTNS gene, resulting in impaired cystine transport and toxic intracellular cystine accumulation. Standard therapy requires lifelong cysteamine treatment, but current formulations are associated with significant gastrointestinal side effects and strict multiple-daily dosing. TTI-0102 has the potential to be dosed once-daily with substantially improved tolerability.
There are an estimated 2,000–2,500 patients with nephropathic cystinosis worldwide. Thiogenesis plans to file an IND in 2026 for a pivotal Phase 3 non-inferiority trial comparing TTI-0102 to existing cysteamine therapies. If successful, TTI-0102 may offer a meaningful improvement in quality of life for patients.
TTI-0102 Key Mechanisms of Action
- Depletes cystine
- Supports glutathione (GSH) generation
- Metabolizes into taurine
About MELAS
Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) is a progressive, multisystem disorder that typically begins in childhood or adolescence. Caused by mutations in mitochondrial DNA—most often m.3243A>G in the MT-TL1 gene—it leads to seizures, muscle weakness, hearing loss, fatigue, and stroke-like episodes.
Despite its severity and an estimated prevalence of 4.1 per 100,000 people, there are currently no approved treatments for MELAS. A Phase 2 clinical trial in the EU was initiated on May 14, 2025.

TTI-0102 Key Mechanisms of Action
- Precursor to glutathione
- Metabolizes into taurine
- Increases the production of CoA
About Leigh Syndrome Spectrum
Leigh syndrome spectrum (“LSS”) is a rare, inherited mitochondrial disorder that typically begins in infancy or early childhood. It is caused by mutations in either mitochondrial DNA or nuclear genes that impair energy production in cells. Symptoms often include developmental delay, loss of motor skills, muscle weakness, breathing difficulties, and lesions in the brainstem and basal ganglia visible on MRI. The condition is progressive and often life-threatening.
The estimated prevalence of LSS is approximately 1 in 40,000 newborns. There are currently no approved therapies for LSS. The Company has received FDA clearance to initiate a Phase 2a clinical trial in the U.S. for the treatment of LSS with TTI-0102.
TTI-0102 Key Mechanisms of Action
- Precursor to glutathione
- Metabolizes into taurine
- Increases the production of CoA
About Pediatric MASH
Pediatric MASH (Metabolic dysfunction-associated steatohepatitis) is a serious liver disease in children that is rapidly escalating; marked by fat accumulation, inflammation, and fibrosis. It is the advanced stage of MASLD (Metabolic dysfunction-associated liver disease) and is closely linked to metabolic syndrome. Oxidative stress plays a central role in its progression by disrupting the balance between reactive oxygen species (ROS) and the liver’s antioxidant defenses.
TTI‑0102 has the potential to restore redox balance by boosting intracellular cysteine and glutathione. It may also reduce liver scarring by inhibiting hepatic stellate cell (“HSC”) activation, which drives fibrosis. There are currently no approved treatments for pediatric MASH. Thiogenesis plans to submit an Investigational Medicinal Product Dossier (IMPD) in 2025 to initiate a Phase 2 clinical trial in Europe.
TTI-0102 Key Mechanisms of Action
- Precursor to glutathione
- Blocks HSC proliferation and fibrosis
- Restores mitochondrial redox balance

